Press Release: CorTec ready for next phase with Brain Interchange System
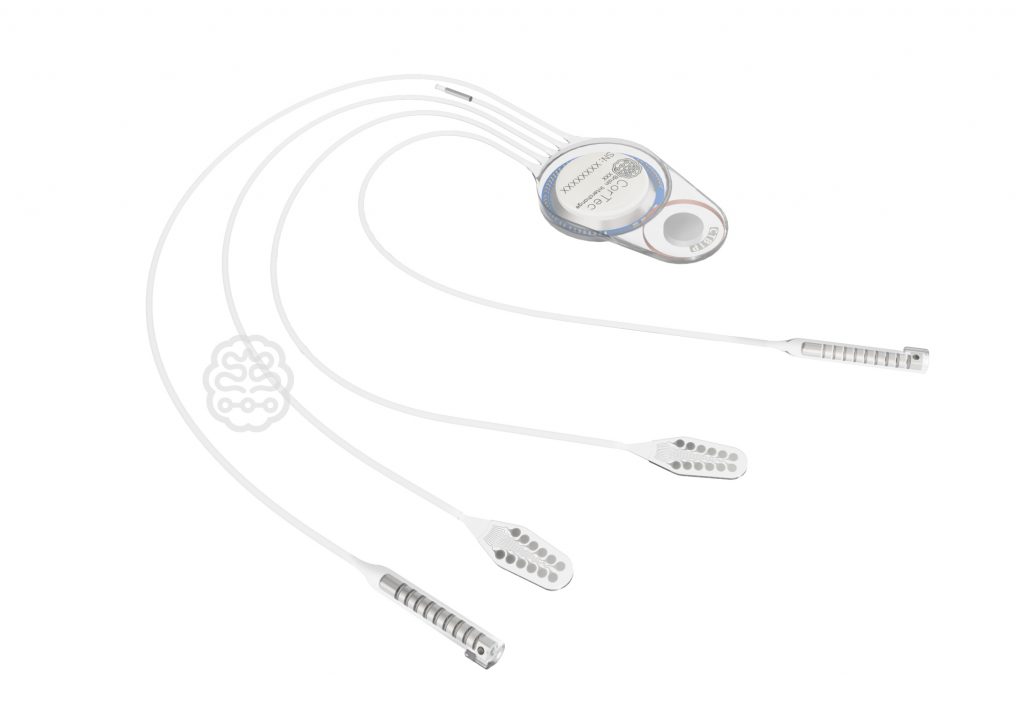
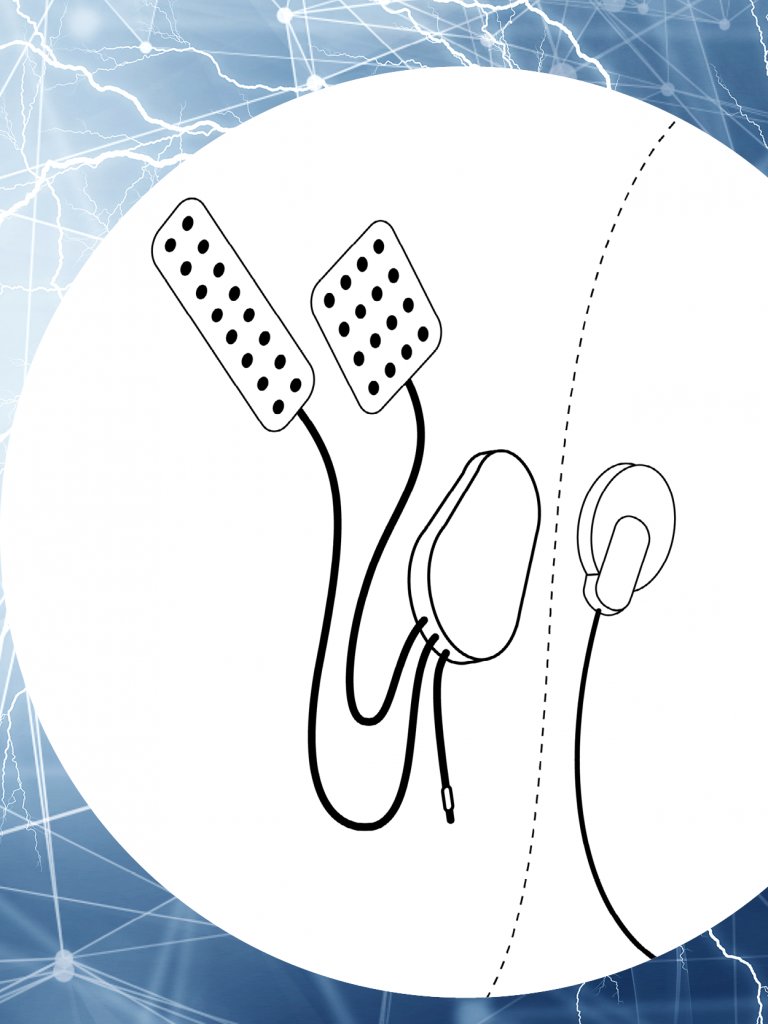
German medical technology innovator poised to make waves after implantation of a brain chip by Neuralink in a human trial As it prepares for the next stage of its groundbreaking device for the treatment of stroke, CorTec GmbH, a pioneering German medical technology company, is poised to make significant strides in the field of neurotechnology. …
Press Release: CorTec ready for next phase with Brain Interchange System Read More »