Brain Interchange One
Brain Interchange One is the first version of the CorTec Brain Interchange Technology which we are currently validating for the use in clinical trials.
On this page you will find more details about the specific product features and design options of this first version.
Device Description
The implantable platform technology features full wireless functionality for chronic open- and closed-loop interaction with the nervous system. It consists of 3 components:

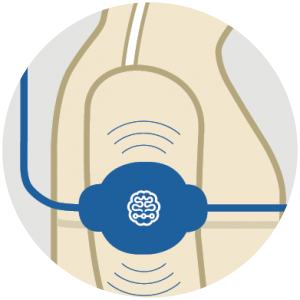
Multi-Part Implant
- One or two of CorTecs °AirRay Electrodes. The Brain Interchange System can be equipped with adapters for DBS electrodes.
- The implanted internal electronics unit is placed inside a proprietary hermetic ceramic encapsulation.
It amplifies, filters and digitizes neural signals and electrically stimulates neural tissue through the electrodes. It is inductively powered by the external unit and communicates with it via a broad-band radio link.

External Unit
- A small, lightweight head piece is held attached to the skin by a magnet opposite to the implant.
- The communication unit for radio communication with the implant, typically belted to the upper arm or wheel chair of the patient, also controls the power supplied to the head piece and communicates with the controller computer.
Personal Computer with Software Interface
- The computer ensures the energy supply of the communication unit.
- It also runs the application software which manages the stream of neural recording data coming from the implant via the external unit.
At this point, innovative experimental algorithms can be implemented which allow a response to the neural data stream.