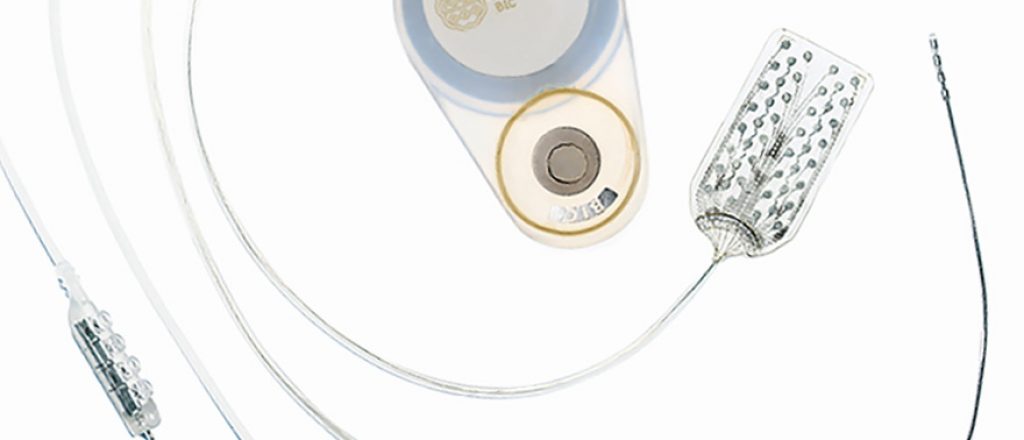
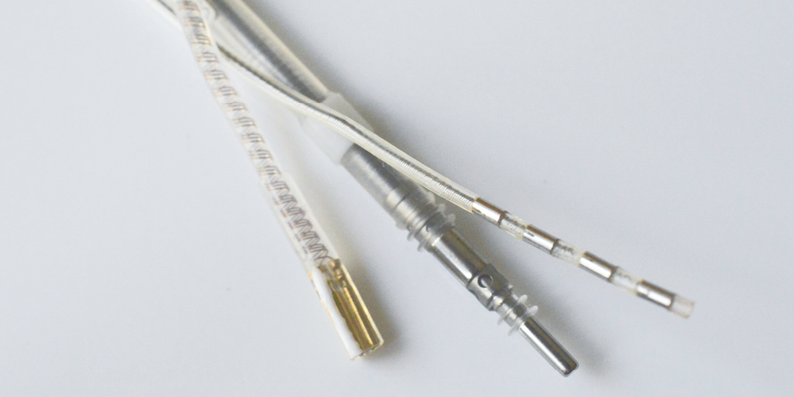
Active Implant Technology
Our hermetic encapsulation technology is the first high channel packaging solution available on the market.
Designed according to your requirements we offer hermetic and non-hermetic encapsulations for the protection of the implant electronics.
The Brain Interchange Technology gives you the opportunity to customize your active implantable device from scratch.
Depending on your specific needs, an individual closed-loop system is developed that fits your individual application.
We are able to provide you with connectivity solutions that are compatible with your individual device design, while fulfilling the industry standards.
Apart from our in-house technologies, we work with a large network of partners. This way we can offer you the full range of solutions according to your requirements.