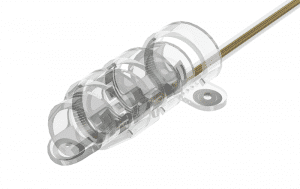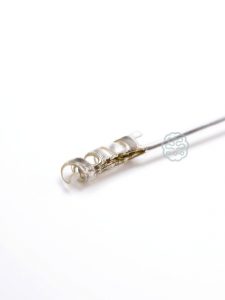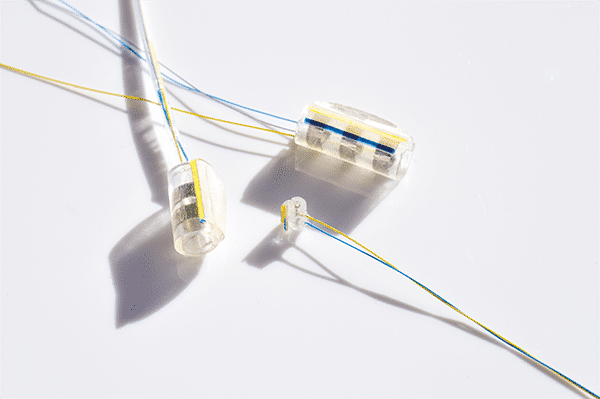
°AirRay Cuff Electrodes
Cuff electrodes are designed to provide an electrical interface to the peripheral nervous system. CorTec has developed several design families to meet the needs of the individual applications, which strongly depend on implantation site (e.g. nerve size) and character of the interface:
• Neural signal recording
• Electrical stimulation of nerves
• Blocking of propagation of neural signals
The cuffs are built from soft silicone to minimize the risk of mechanical nerve irritation. CorTec‘s micromachining technology permits the electrical contacts to be produced as micro meanders which are very flexible. At the same time they prevent the effect of plastic deformation of the cuffs during handling. The following page presents typical design options to these electrodes. Of course, we also offer custom-tailored designs to meet your specitic requirements, e.g. high-channel versions. Please do not hesitate to contact us if you want to discuss individual solutions for your next project.
Tunnel Cuff
- for nerve diameters 0.15-6.0 mm
- easy to handle, double sealing lips
- flexible meander-ring structured contacts
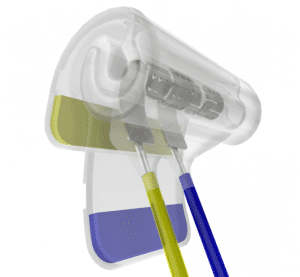

Sling Cuff
- for nerve diameters 0.1-0.5 mm
- very small nerves
- buckle-and-belt closure
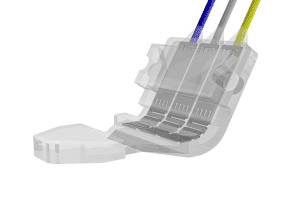
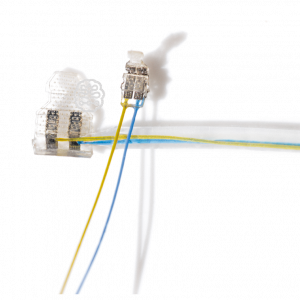
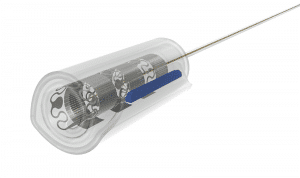
Spiral Cuff
- for nerve diameters 1.5-6.0 mm
- self-adjusting diameter
- ideal for chronic use
- accommodates nerve swelling to some extent
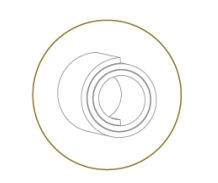
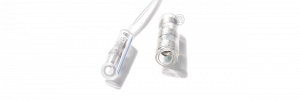
Helix Cuff
- for nerve diamters 1.5-6.0 mm
- self-adjusting diameter
- ideal for chronic use
- accommodates nerve swelling to some extent